Raleigh, NC: (919) 277-9299
About Bard Port Catheters
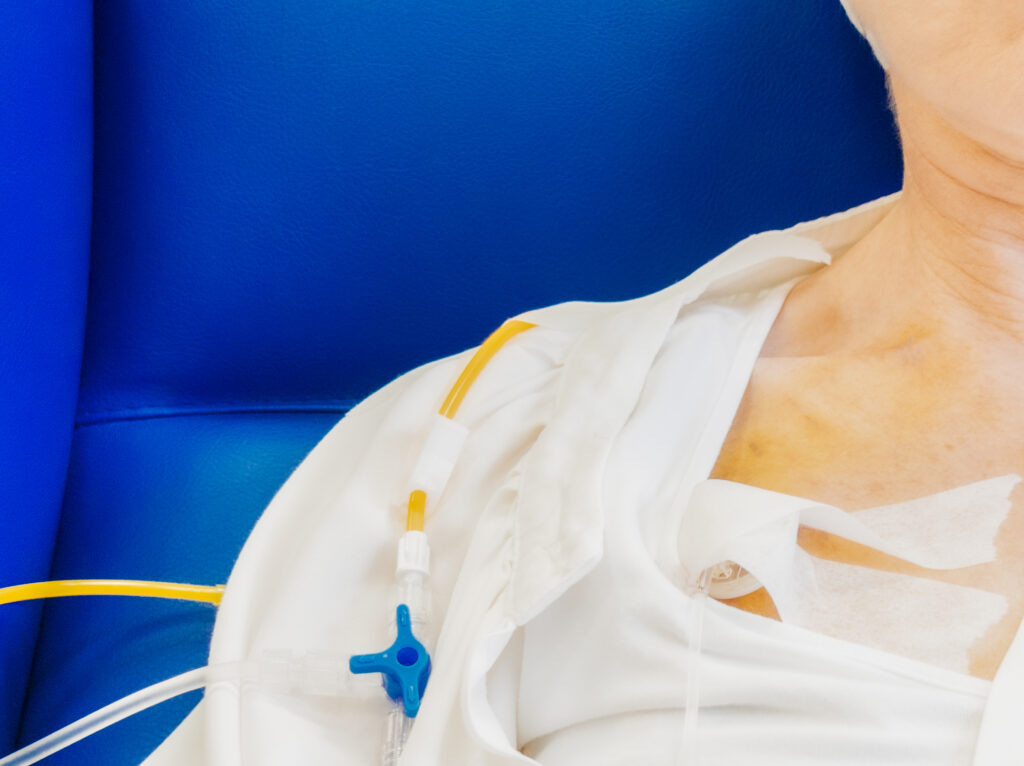
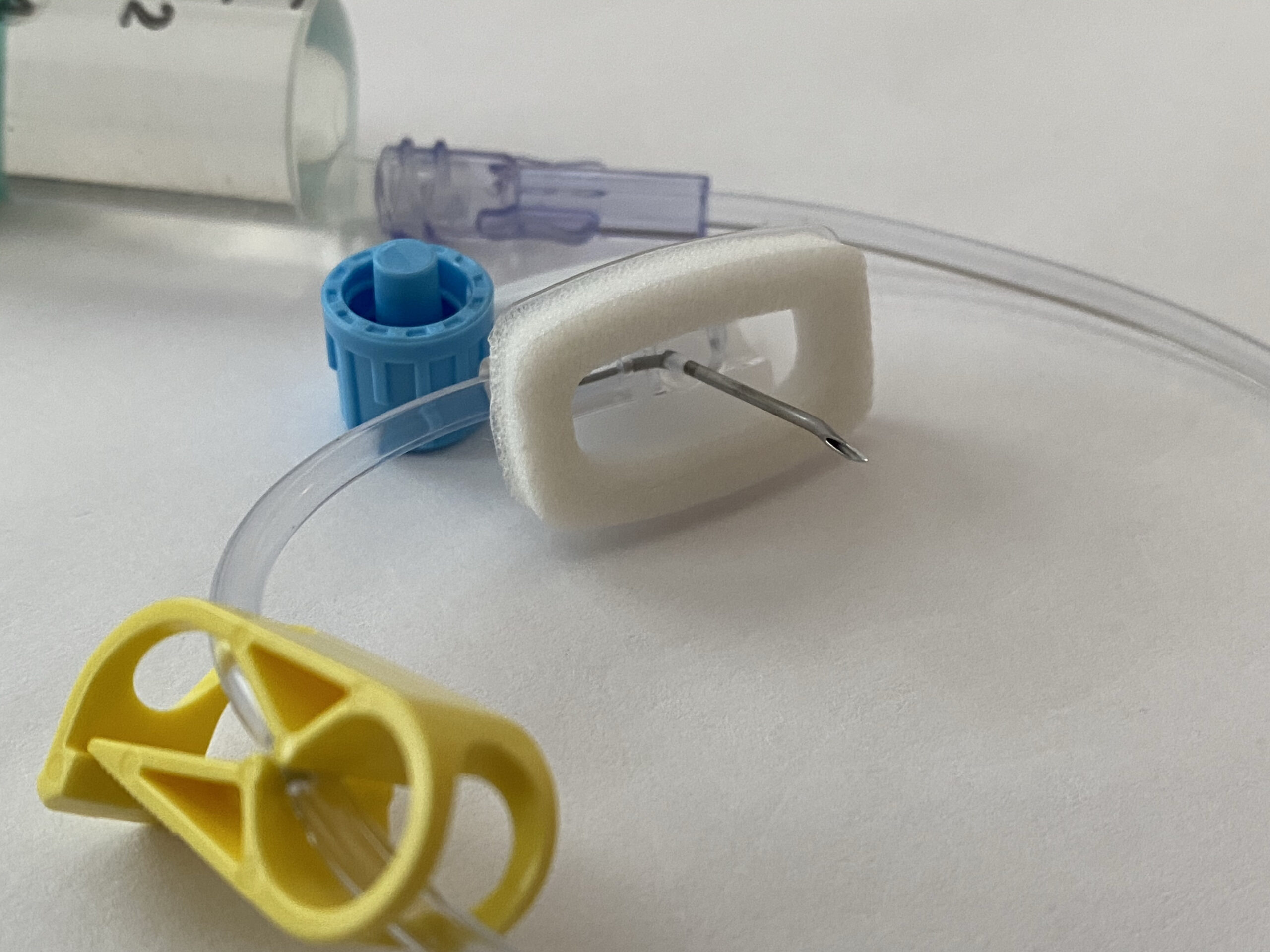
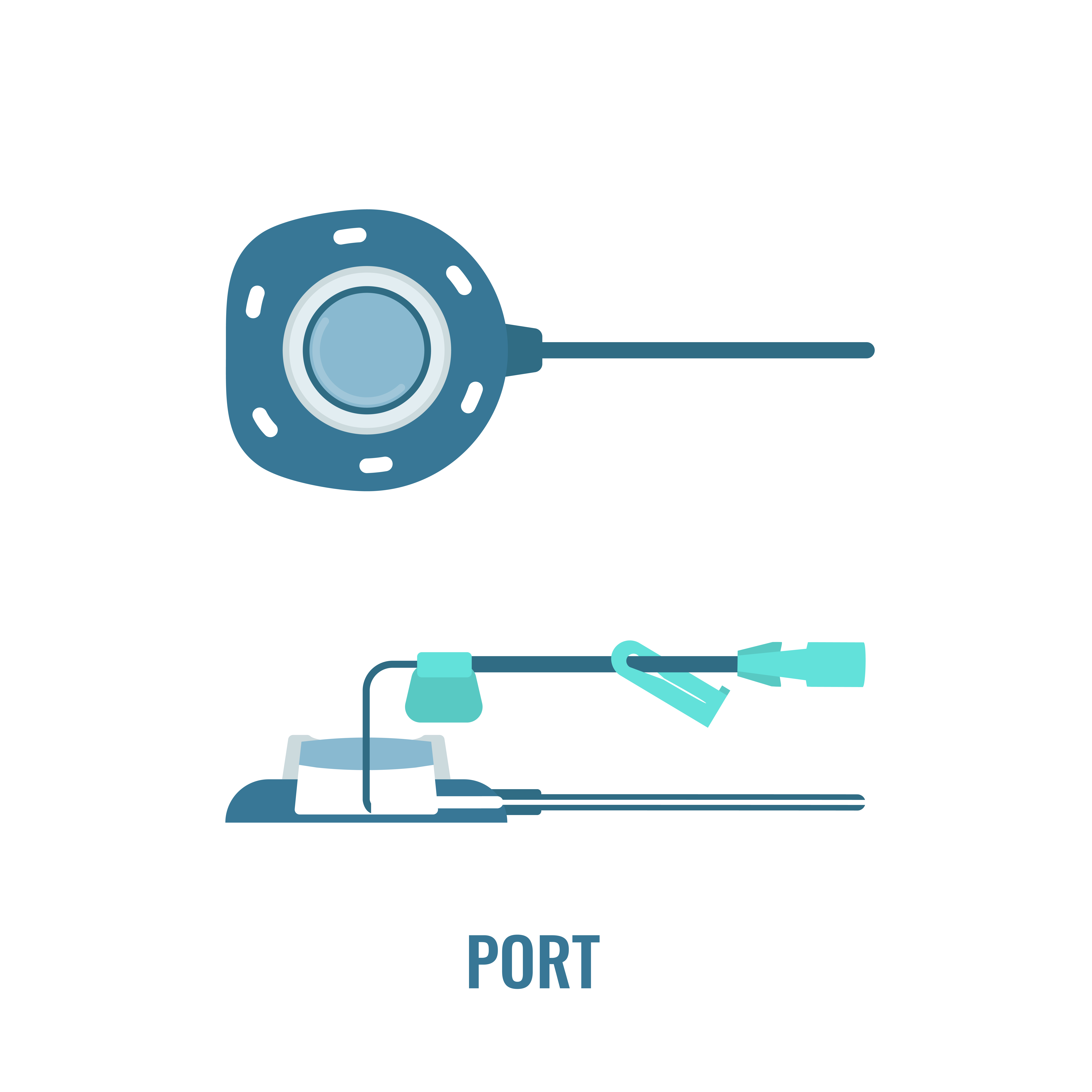
Bard PowerPort catheters are implantable devices used to provide repeated access to a patient’s veins. They are often used for patients requiring long-term intravenous (IV) treatments, like chemotherapy.
While all implantable ports may come with risks, lawsuits claim there are design elements specific to Bard PowerPort devices that may increase the likelihood of the device breaking or migrating.
The Basis for the PowerPort Liability Lawsuit
Medical device manufacturers are responsible for creating products that work as intended and meet consumer safety standards. Lawsuits allege Bard ignored these duties in one or more of the following ways:
Enough lawsuits were pending against Bard for its defective PowerPort products that the Federal Judicial Panel centralized these claims into a multi-district litigation (MDL). Though the legal proceedings are held in the US District Court of Arizona, claimants may file with any product liability attorneys they choose, including Bell Legal Group. The purpose of an MDL is to expedite discovery and pre-trial processes without losing the individual merits of each claim.
Bard PowerPort Defects and Injuries
These are the three main injuries of individuals filing Bard PowerPort lawsuits:
When these devices fail, the consequences can be devastating. Recovering from injuries due to a defective catheter can require additional medical procedures, followup care, hospital stays, and ongoing infection control. Some injuries may be fatal.
If a loved one succumbed to the effects of contaminated water exposure at Camp Lejeune, you might be eligible to pursue a claim to secure compensation on their behalf. The Bell Legal Group Lejeune Team℠ can assess whether you have the legal standing to initiate such a claim and guide you through the process.
Who Is Eligible To File a Bard PowerPort Lawsuit?
You may be eligible to file a Bard PowerPort lawsuit if you or a loved one:
Don’t delay getting help if you believe you qualify to file a complaint against Bard for harm caused by any of its faulty catheter devices. The statute of limitations varies by state and many factors can influence the timeline. If this deadline lapses, you’ll lose your opportunity to hold this negligent manufacturer responsible.
Devices Involved in the PowerPort Lawsuit
The defective catheters produced by Bard are part of the ‘PowerPort’ line of devices—known as BardPorts, for short. The lawsuits involve several PowerPort models and styles, including:
This isn’t an exhaustive list of models or brands so it’s important to speak to one of our attorneys to confirm whether this MDL covers the Bard PowerPort that harmed you.
Contact our team of product liability attorneys online or call (843) 438-7480 to verify your eligibility and begin your recovery. We’re ready to help!